STP stands for Standard Temperature and Pressure. Nowadays the STP is temperature of 0°C (273.15K) and pressure of 1 bar.
Assuming the gas behaves as an ideal gas, in these conditions the volume of each mol of gas is approximately 22.7 L.
Using rule of three, we can calculate the volume of 0.001 mol of H₂S:
Volume --- Number of moles
x --- 0.001 mol
22.7L --- 1 mol
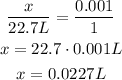
So, the volume is approximately 0.0227 L.