Given,
The initial pressure of the gas, P₁=8.5 kPa
The initial volume of the gas, V₁=2.4 m³
The initial temperature of the gas, T₁=10 °C=282.15 K
The temperature of the building, i.e., the temperature of the gas after entering the building, T₂=35 °C=308.15 K
The pressure of the gas after entering the building, P₂=2 kPa
Let us assume the new volume of the gas is V₂
From the combined gas law,
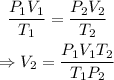
On substituting the known values,
![undefined]()