We have the concentration of H+ ions, from these concentrations we can find the pH values of the solutions with the following equation:
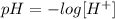
Where [H+] is the concentration of H+ ions.
Now, the acidity or basicity of a solution depends on the pH value. A solution above 7 will be basic, below 7 will be acidic, and equal to 7 will be neutral.
Let's determine the pH value for each solution:
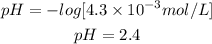
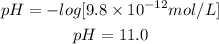
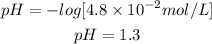
So, we have that:
A. [H+] = 4.3 × 10-3 mol/L. pH=2.4. Acidic
B. [H+] = 9.8 × 10-12 mol/L pH = 11.0 Basic
C. [OH-] = 4.8 × 10-2 mol/L pH=1.3 Acidic