
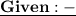
- We have 250g of liquid water and it needs to be cool at temperature from 100° C to 0° C
- Specific heat of water is 4.180J/g°C
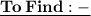
- We have to find the total number of joules released.
We know that,
Amount of heat energy = mass * specific heat * change in temperature
That is,
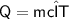
Subsitute the required values in the above formula :-
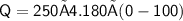
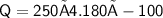
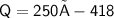
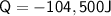
Hence, 104,500 J of heat is released to cool 250 grams of liquid water from 100° C to 0° C.
We have to tell whether the above process is endothermic or exothermic :-
Here, In the above process ΔT is negative and as a result of it Q is also negative that means above process is Exothermic
- Exothermic process :- It is the process in which heat is evolved .
- Endothermic process :- It is the process in which heat is absorbed .