Answer
19.6 grams
Step-by-step explanation
Given:
Mass of calcium phosphate produced = 50.500 g
Equation: 3Ca(s) + 2H3PO4(aq) ---> Ca3(PO4)2(s) + 3H2(g)
What to find:
The grams of calcium required to produce 50.500 g of calcium phosphate.
Step-by-step solution:
From the equation, 3 mol Ca produce 1 mol Ca3(PO4)2
1 mole Ca3(PO4)2 = 310.19 grams
1 mole Ca = 40.078 grams
This means, (3 x 40.078 g) = 120.234 g Ca produce 310.19 g Ca3(PO4)2
So x grams Ca will be required to produce 50.500 grams Ca3(PO4)2
x grams Ca will be equal
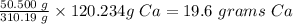
The grams of calcium required to produce 50.500 g of calcium phosphate = 19.6 grams