In order to calculate the temperature, we need to know that temperature and pressure are directly proportional, that is, if the pressure increases, the temperature (in Kelvin) also increases in the same proportion.
So, first let's convert the temperature from Celsius to Kelvin, by adding 273 units:
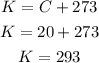
Then, let's calculate the proportion:
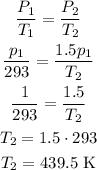
Now, converting back to Celsius, we have:
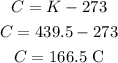
So the temperature would be 166.5 °C.