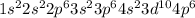The atomic number of rubidium is 37, it means that its electron configuration is as follows:
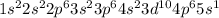
Rubidium is an alkali metal. Its ion is formed when it loses an electron. It means that the rubidium ion has 36 electrons instead of 37, and its configuration is as follows: