Step-by-step explanation:
We are given: mass of FePO4 = 15g
: mass of Na2SO4 = 5g
We first find the mass of Fe2(SO4)3 from the mass of FePO4:
m is the mass and M is the molar mass
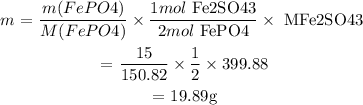
We then find the mass of Fe2(SO4)3 from Na2SO4:
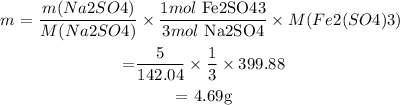
Answer:
Therefore, FePO4 is the excess reactant and Na2SO4 is the limiting reactant