Given:
Mass, m = 0.68 kg
Initial temperature, T1 = 20°C
Final temperature, T2 = 45°C
Let's find the amount of heat needed.
Apply the Specific Heat Capacity formula:
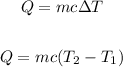
Where:
m is the mass = 0.68 kg
c is the specific heat of water = 4.187 J/kg °C−1
T1 = 20°C
T2 = 45°C
Plug in the values and solve for Q:
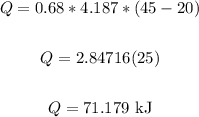
Where:
1 kJ = 0.239 kCal
Since the answer is to be in kCal, we have:
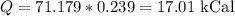
Therefore, the amount if heat added is 17.01 kCal.
ANSWER:
17.01 kCal