Answer
a. C₂H₂O + 2O₂ → 2CO₂ + H₂O
b. 8 moles of O₂
c. 64 g of O₂
d. The grams of CO₂ produced is 262 g and the mass of H₂O produced is 53.6 g.
Step-by-step explanation
a. The balanced chemical equation gasohol is a fuel containing liquid ethanol (C₂H₂O) that burns in oxygen gas to give carbon dioxide and water gases is:
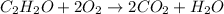
b. The moles of O₂ needed to completely react with 4.0 moles of C₂H₂O?.
The mole ratio of O₂ to C₂H₂O in the balanced equation is 2:1.
Therefore, 4.0 moles of C₂H₂O will completely react with (2 x 4) = 8 moles of O₂.
c. The mass in grams of O₂ that is used up in the reaction if a car produces 88 g of CO₂.
The molar mass of CO₂ = 44.01 g/mol
The molar mass of O₂ = 31.999 g/mol
The mole ratio of O₂ to CO₂ is 2:2
This implies (2 mol x 31.999 g/mol) = 63.998 g of O₂ is used up to produce (2 mol x 44.01 g/mol) = 88.02 g of CO₂
Therefore x g of O₂ will be used up to produce 88 g of CO₂
That is;
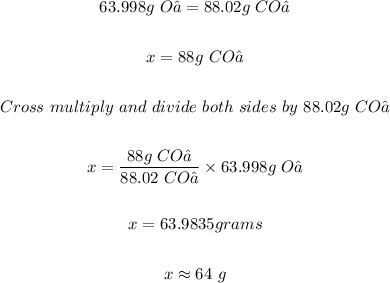
Hence, the mass in grams of O₂ that is used up in the reaction if a car produces 88 g of CO₂ is 64 g.
d. The grams of CO₂ and H₂O that can be produced if 125 g of C₂H₂O were burnt.
1 mole of C₂H₂O = 42.04 g
1 mole of CO₂ = 44.01 g
1 mole of H₂O = 18.01 g
Mass of CO₂ produced:
From the balanced, the mole ratio of C₂H₂O to CO₂ is 1:2
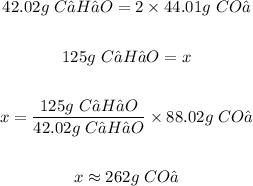
The grams of CO₂ produced is 262 g.
Mass of H₂O produced:
From the balanced, the mole ratio of C₂H₂O to H₂O is 1:1
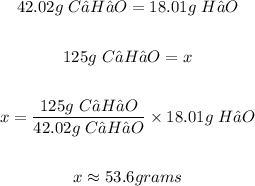
The mass of H₂O produced is 53.6 g