Write the balanced chemical equation for the reaction as follows:
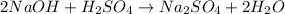
Given:
Concentration of sodium hydroxide =1.50 mol/L
Volume of sodium hydroxide = 20 mL
Concentration of sulfuric acid = x
Volume of sulfuric acid = 20 mL
Firstly, we will determine the moles that is in 20 mL of 1.5 M NaOH:
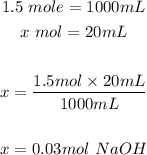
Based on the balanced chemical equation and the moles of NaOH we can use stoichiometry (ratios) to determine the moles of 20 mL sulfuric acid:
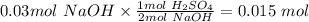
Now that we know how many moles are in 20 mL of H2SO4, we can determine the moles in 1000mL (initial concentration):
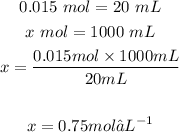
Answer: D) 0.75 mol/L,