1) Write the chemical equation.
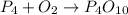
2) List the elements in the reactants and in the products.
Reactants.
P: 4
O: 2
Products
P: 4
O: 10
Balance O.
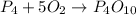
3) Moles of O2 needed.
The molar ratio between P4 and O2 is 1 mol P4: 5 mol O2.
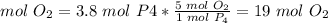
If 3.8 mol P4 are used, 19 mol O2 are needed.
.