Step 1
Charles's law states that the volume (V) of a gas is directly proportional to its absolute temperature. It is assumed that the pressure and the quantity of gas remain constant.
Mathematically:
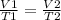
-------------------
Step 2
Information provided:
T1 = 10 °C + 273 = 283 K
V1 = 3 L
---
T2 = Unknown
V2 = 10 L
-------------------
Step 3
Procedure:
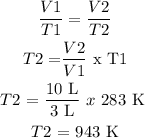
Answer: 943 K or 670 °C