Answer
B) 47.0
Step-by-step explanation
If at STP, exactly 1 mole of any gas occupies 22.4 L
Then, 2.1 moles of O₂ gas at STP will occupy x L container
To get x L, cross multiply and divide both sides by 1 mole.
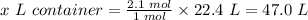
Therefore, the size of the container (in Liters) needed to hold 2.1 mol O₂ gas at STP is 47.0 L