1) List the known and unknown quantities.
First experiment:
Reactants
Phosphorus: 7.97 g.
Bromine: excess.
Product
Phosphorus tribromide: 69.65 g.
Second experiment
Reactants
Phosphorus: 12.05 g.
Bromine: 61.68 g.
Product
Phosphorus tribromide: unknown.
2) Write and balance the chemical equation.
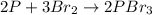
3) Convert the masses.
3.1-Convert the mass of P to moles of P.
The molar mass of P is 30.97 g/mol.
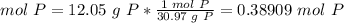
3.2-Convert the mass of Br to moles of Br.
The molar mass of Br2 is 159.8080 g/mol.
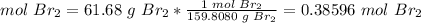
4) Which is the limiting reactant?
4.1-How many moles of Br2 do we need to use all of the P?
The molar ratio between Br2 and P is 3 mol Br2: 2 mol P.
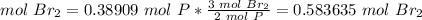
We need 0.583635 mol Br2 and we have 0.38596 mol Br2. We do not have enough Br2. This is the limiting reactant.
4.2-How many moles of P do we need to use all of the Br2?
The molar ratio between Br2 and P is 3 mol Br2: 2 mol P.
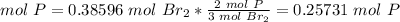
We need 0.25731 mol P and we have 0.38909 mol P. We have enough P. This is the excess reactant.
5) Moles of phosphorus tribromide produced from the limiting reactant.
Limiting reactant: 0.38596 mol Br2.
The molar ratio between Br2 and PBr3 is 3 mol Br2: 2 mol PBr2.
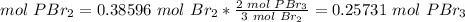
6) Conver the moles of Pbr3 to mass of PBr3.
The molar mass of PBr3 is 270.6858 g/mol.
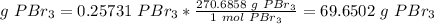
The mass of PBr3 produced in the reaction is 69.65 g PBr3.
.