The question requires us to calculate the number of moles that corresponds to 5.01 x 10^22 atoms of magnesium.
To solve this question, we'll need to use the Avogadro constant. This number, defined as 6.02 x 10^23, relates the number of constituent particles (molecules, atoms or ions, for example) in a sample with the amount of substance in that sample. We can use it by saying that 1 mol of a substance corresponds to 6.02 x 10^23 constituent particles of this substance.
Then, using the information above, we can calculate the number of moles in 5.01 x 10^22 atoms of magnesium (Mg):
6.02 x 10^23 atoms of Mg --------------- 1 mol of Mg
5.01 x 10^22 atoms of Mg ---------------- x
Solving for x, we'll have:
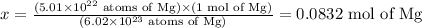
Therefore, 5.01 x 10^22 atoms of magnesium corresponds to 0.0832 mol of this element.