First, we have to remember what a mass percentage means (in this particular case):
It is the amount of each element in a compound, and it is calculated as follows:
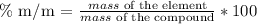
The addition of the different mass percentages is equal to 100%.
In this case, we have the percentage of one of the elements of the compound, we can make the respective subtraction:
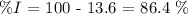
Then, the answer is that the iodine mass percentage is 86.4%