The heat absorbed or released by a substance is given by the following formula:
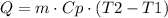
Where Q is the heat absorbed or released, m is the mass of the substance, Cp is the specific heat, T2 is the final temperature and T1 is the initial one.
We know the values of Q, m, T2 and T1 because they are given by the question statement, and we have to find the value of Cp.
Solve the equation for Cp and use the given values to find its value:
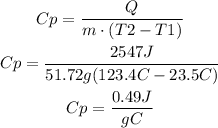
The specific heat of the substance is 0.49J/g°C.