Answer:
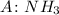
Step-by-step explanation:
Here, we want to get which of the compounds have the highest percentage by mass of nitrogen
What we have to do here is to divide the mass of nitrogen atoms in the compound by the molecular mass of the compound
a) NH3
The atomic mass of nitrogen is 14 amu, the atomic mass of hydrogen is 1 amu
The molar mass is thus 14 + 3(1) = 17 g/mol
The percentage by mass of nitrogen will be
14/17 * 100 = 82.35 %
b) HCN
The atomic mass of carbon is 12 amu
Thus, we have the molar mass as
1 + 12 + 14 = 27 g/mol
Percentage of nitrogen by mass will be : 14/27 * 100 = 51.85 %
c) For N2O
The atomic mass of oxygen is 16 amu
The molar mass of the compound will be 14(2) + 16 = 44 g/mol
Percentage of nitrogen by mass will be = 28/44 * 100 = 63.64 %
d) NI3
The atomic mass of iodine is 127 g/mol
So the molar mass of the compound will be:
14 + 3(127) = 395 g/mol
Percentage by mass of nutrogen will be
14/395 * 100 = 3.54 %
From what we have, the highest percentage by mass of nitrogen is 82.35 % and that belongs to ammonia (NH3)