The reaction to produce nitrogen dioxide from nitrogen and oxygen is as follows:
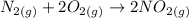
Since all the reactants and products are gases, we can take the coefficients as liters of substance.
Dividing the volumes we have of each reactant by their corresponding coefficient we can determine the limit reagent:
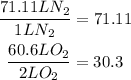
From this we can conclude that the limit reagent is oxygen.
We have to base our calculations on this substance.
According to the given equation, 2 L of O2 produce 2 L of NO2, use this ratio to find the volume of nitrogen dioxide produced:
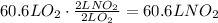
It means that 60.6 liters of nitrogen dioxide gas are made.