We must first check the valence number of the sulfur in the sulfate ion. In this ion, the valence number is +6 and we have 4 bonded oxygens.
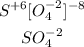
The Lewis structure of the sulfate ion is as follows:
We have six bonding in the molecule. So the answer will be B. six bonding and no lone pairs of electrons.