They give us the grams of solute and the molarity of the solution. To find the liters we must take into account the definition of molarity. Molarity is defined as:
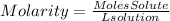
The definition of molarity asks us for the moles of solute, so we must pass the grams that give us to moles. For them, we divide the 500 grams by the molar mass of MgF2. The molar mass of MgF2 is 62.3018g/mol.
The moles of MgF2 will be:
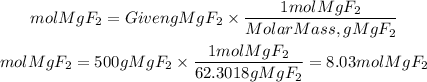
Now, we find the liters of solution:

Answer: It must be present 3L of solution