This organic compound has in its structure the next element: C, H, and O
45.27% C
4.43 % H
The rest 100%-45.27%-4.43 = 50.3 % O
-----------------------------------------------------------------------------------------------------------------
The empirical formula of a chemical compound is the simplest whole-number ratio of atoms present in a compound.
Procedure:
Step 1)
We convert % into grams (g). Let's assume we have a 100g sample. Therefore,
45.27 % C = 45.27 g
4.43 % H = 4.43 g
50.3 % O = 50.3 g
Step 2)
We calculate the number of moles of each element. To do this, we need every atomic mass
For C)
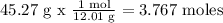
For H)
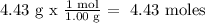
For O)
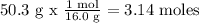
Step 3)
We divide all moles by the smallest one of them (3.14 moles of O)
For C) 3.767 moles/3.14 moles = 1.19 = 1 (we need integer numbers)
For H) 4.43 moles/3.14 moles = 1.41 = 1
For O) 3.14 moles/3.14moles = 1
The empirical formula is CHO