ANSWER
The mass of Na2O in grams is 123.49 grams
Step-by-step explanation:
Given information
The mass of sodium is 91 grams
The mass of oxygen is 34.0 grams
To find the amount of Na2O produced, follow the steps below
Step 1: Write the balanced equation of the reaction
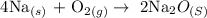
Step 2: Find the number of moles of sodium and oxygen using the formula below
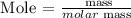
Recall, that the molar mass of Na is 22.989 g/mol, and the molar mass of oxygen atom is 32 g/mol
For Na
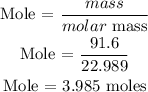
For O2
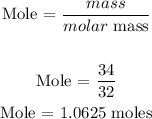
Step 3: Find the limiting reactant of the reaction
The limiting reactant is the reactant that has the least number of moles after dividing it by the coefficient of the reactant
From the reaction above, you will see that 4 moles of sodium react with one mole of oxygen to give 2 moles of sodium oxide.
For Na
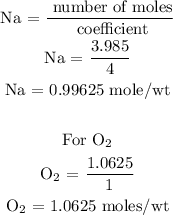
From the above calculations, you will see that Na has the least number of moles, hence, Na is the limiting reactant
Step 4: Find the number of moles of Na2O
The Na2O can be determined using a stoichiometric ratio
Let x represents the number of moles
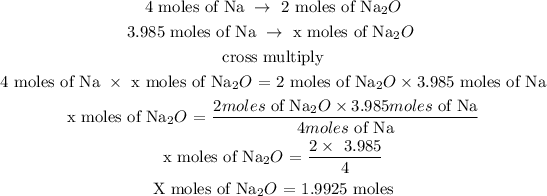
Hence, the number of moles of Na2O is 1.9925 moles
Step 5: Find the mass of Na2O
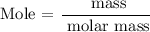
Recall, that the molar mass of Na2O is 61.9789 g/mol
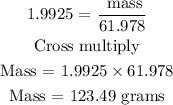
Hence, the mass of Na2O in grams is 123.49 grams