Cl is the limiting reactant and the mass of tin chloride that can be formed is 31.9g.
- First, we need to balance the equation:
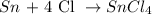
- Second, we need to know the molar weight of the compounds:
Sn: 118.7 g/mol
Cl: 35.5 g/mol
SnCl4: 260.7 g/mol
- Third, we need to know how much Sn can react with Cl according to the balanced equation:
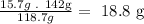
If 118.7g of Sn needs 142g of Cl to react according to the balanced equation, we found that the 15.7g of Sn will need 18.8g of Cl to complete the reaction.
As we have 17.4g of Cl available, we can see that we are going to need more Cl and therefore Cl is the limiting reactant.
- Now, to find the mass of tin chloride that can be formed, we use the limiting reactant amount for the calculation:
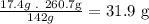
According to the balanced equation we need 142g of Cl to produce 260.7g of tin chloride, so with 17.4g of chlorine we will obtain 260.7g of SnCl4.
So, the mass of tin chloride that can be formed is 31.9g.