To solve this problem we can use the Ideal gas law:
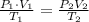
The problem give us de following information:
P1= 131kPa
V1=1.82L
T1=-30°C= 243.15 K
P2= 233 kPa
V2= 1.3L
Then we just have to solve for T2 and use the information provided:
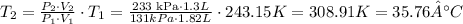
Then the answer is T2=35.76°C