Answer:
5998kJ
Explanations:
Given the reaction between ethanol and oxygen expressed as:

For the product
We have 2 moles of CO2 and 3 moles of water. The total bond energy in the product is expressed as:
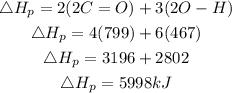
Hence the total energy transferred to build all the bond energy in the product is 5998kJ