Given:
Dimensions and weight of a solid is given.
Height (h) of a cylinder (in cm) =
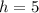
Radius (r) of a cylinder (in cm) =
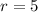
Mass (m) of solid (in grams)=
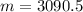
Density of several elements is given.
Cobalt=8.86, Copper=8.96, Gold=19.3, Iron=7.87, Lead 11.3, Platinum=21.5, Silver=10.5, Nickel=8.90.
Required:
What element is the solid made up of.
Answer:
Let us find the volume (V) of cylinder (in cubic cm).
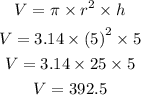
Using formula of density (D), we get,
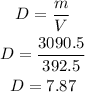
Hence, the density of the solid is 7.87 grams per cubic cm.
From the given information of density of several elements, we see that the solid is made up of Iron.
Final Answer:
The solid is made up of Iron.