Answer
The percent yield = 83.36%
Step-by-step explanation
Given:
Experimental yield = actual yield = 15.68 g
Theoretical yield = 18.81 g
What to find:
The percent yield for the reaction.
Step-by-step solution:
The percent yield for the reaction can be calculated using the formula below:
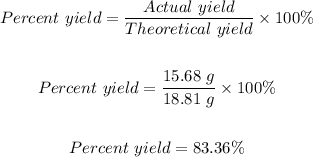
Hence, the percent yield for the reaction is 83.36%