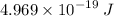Answer:
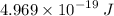
Step-by-step explanation:
The energy of the purple lamp can be found by using the formula
E = hf
where
E is the energy
f is the frequency
h is the Planck's constant which is
6.626 × 10-³⁴ Js
From the question.
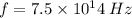
We have.
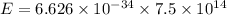
We have the final answer as