Answer:
No. of moles
 0.020
0.020
Step-by-step explanation:
To calculate the number of moles of a substance in a solution given its concentration and volume, we use the following formula:
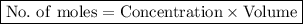 ,
,
where concentration is in mol/dm³ (or M) and volume is in L.
In the question, we are told that the volume of the solution is 0.085 L and that its concentration is 0.23 M. Substituting these values into the formula above, we get:
No. of moles = 0.23 × 0.085
= 0.01955
 0.020 mol (3 s.f.)
0.020 mol (3 s.f.)
Therefore, there are 0.020 moles of NH₄NO₃ in the solution.