Answer:
2.1 m³
Step-by-step explanation:
Boyle's Law states:
The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system (Wikipedia) }
We can express this relationship as
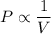
where V is the volume under pressure P
This can be re-written as:
PV = a constant
We are given that
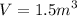 when
when
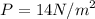
So PV = 1.5 x 14 = 21 Nm
At
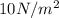 the PV product must also be 21
the PV product must also be 21
So 10V' = 21 where V' = volume at
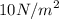
Therefore V' = 21/10 = 2.1 m³