Let's use the variable x to represent the amount of pure acid and y to represent the amount of 10% acid.
Since the total amount wanted is 90 L, we can write the equation:
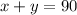
Also, the final solution is 81%, so we can write our second equation:
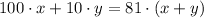
From the first equation, we can solve for y and we will have y = 90 - x.
Using this value in the second equation, we have:
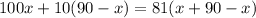
Solving for x, we have:
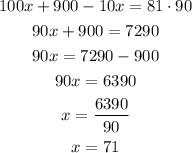
Therefore the amount of pure acid to be used is 71 L and the amount of 10% acid is 19 L.