Solving with Moles
Mole equation:
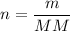
- n = number of moles (mol)
- m = mass (g)
- MM = molar mass (g/mol)
To find mass given number of atoms:
- Divide number of atoms by the number of atoms in the chemical formula ⇒ find number of molecules
- Divide number of molecules by Avogadro's number (
 ) ⇒ find number of moles (n)
) ⇒ find number of moles (n) - Solve for m using moles equation
Solving the Question
We're given:
- Ag (silver)
- Atoms =
 atoms
atoms - m = ?
In the chemical formula, which is Ag, there is only 1 atom.
Divide
 atoms by 1 atom to get the number of molecules in the silver ring:
atoms by 1 atom to get the number of molecules in the silver ring:

Therefore, there are
 molecules in the silver ring.
molecules in the silver ring.
Now, divide
 molecules by Avogadro's number to find n:
molecules by Avogadro's number to find n:

Therefore, the sample has 0.30066 mol.
Finally, solve for the mass using the moles equation:
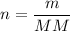
⇒ Rearrange the equation:
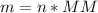
⇒ MM of Ag = 107.87 g/mol
⇒ Plug in given information:
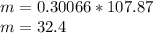
Therefore, the mass of the ring is 32.4 g.
Answer
32.4 g