Answer:
19.5°C (nearest tenth)
Step-by-step explanation:
When a gas is trapped inside a container whose volume cannot change, the pressure is directly proportional to the Kelvin temperature.
Gay Lussac's Law
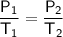
where:
- P₁ is the initial pressure.
- T₁ is the initial temperature.
- P₂ is the final pressure.
- T₂ is the final temperature.
Temperature must be in kelvins (K).
To convert Celsius to kelvins, add 273.15.
Given values:
- P₁ = 4.882 atm
- P₂ = 4.690 atm
- T₂ = 8 C = 8 + 273.15 = 281.15 K
Substitute the given values into the equation and solve for T₁:
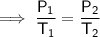
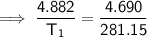
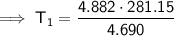
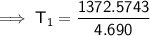
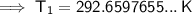
To convert kelvins to Celsius, subtract 273.15:
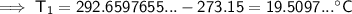
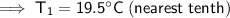
Therefore, the temperature of the previous day was 19.5°C (nearest tenth).