Final answer:
Sodium hydroxide (NaOH) is the base in the reaction NaOH(s) + H2O(l) → N
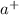 (aq) + OH-(aq), as it releases hydroxide ions (O
(aq) + OH-(aq), as it releases hydroxide ions (O
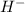 ) when dissolved in water, making it a strong base.
) when dissolved in water, making it a strong base.
Step-by-step explanation:
When we look at the reaction NaOH(s) + H2O(l) → N
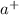 q) + O
q) + O
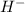 q), we can identify the base in the reaction. A base is known to release hydroxide ions (O
q), we can identify the base in the reaction. A base is known to release hydroxide ions (O
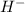 ) when dissolved in water. In this reaction, sodium hydroxide – NaOH is the compound that dissociates to yield the sodium cation (N
) when dissolved in water. In this reaction, sodium hydroxide – NaOH is the compound that dissociates to yield the sodium cation (N
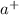 ) and the hydroxide anion (O
) and the hydroxide anion (O
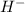 ). Therefore, NaOH is the base in this reaction. It is a strong base, as it releases hydroxide ions very easily into the solution. Additionally, as per the Arrhenius definition of a base, NaOH fits the criteria since it increases the concentration of OH- ions in the solution upon dissolution.
). Therefore, NaOH is the base in this reaction. It is a strong base, as it releases hydroxide ions very easily into the solution. Additionally, as per the Arrhenius definition of a base, NaOH fits the criteria since it increases the concentration of OH- ions in the solution upon dissolution.