Answer:
Energy = 3.77 × 10⁻²⁸ J; radio wave.
Step-by-step explanation:
In order to calculate the energy of an electromagnetic wave given its frequency, we have to use the following formula:
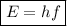 ,
,
where:
• E = energy of wave
• h = Planck's constant (6.63 × 10⁻³⁴ m² kg / s)
• f = frequency of wave (5.68 × 10⁵ Hz)
Using the formula and information above, we can calculate the energy of the wave:
E = 6.63 × 10⁻³⁴ × 5.68 × 10⁵
= 3.77 × 10⁻²⁸ J
Therefore, the energy of the wave is 3.77 × 10⁻²⁸ J, and it is a radio wave, as radio waves have frequencies of magnitude 10⁴ to 10⁵ Hz.