Answer:
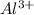 is more common
is more common
Step-by-step explanation:
To see if an ion is stable, check to see if it has an empty valance S orbital, an empty P orbital, or a half-full P orbital. Aluminium has an empty S and P orbital, so it is stable. On the other hand, Sodium 3+ has 4 electron in the P orbital. Sodium also only has one valance electron, and to make a +3 ion, two core electrons in addition to that valance electron has to be removed. Core electrons are really hard to remove, so any ion that requires a core electron being removed is quite unstable.