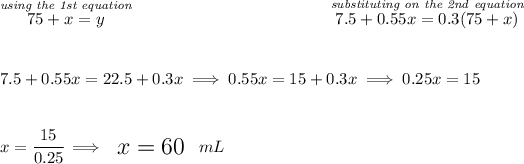A = 10% alcohol solution
B = 55% alcohol solution
we know we have 75 mL of A, that means A itself since it's 10% alcohol, really only has 75 * (10/100) = 7.5 mL of alcohol, likewise the B solution has say "x" mL, so alcohol wise it only has "x" * (55/100) = 0.55x, and we have a final mixture we need of say "y" mL at 30% alcohol.
![\begin{array}{lcccl} &\stackrel{solution}{quantity}&\stackrel{\textit{\% of }}{amount}&\stackrel{\textit{mL of }}{amount}\\ \cline{2-4}&\\ A&75&0.10&7.5\\ B&x&0.55&0.55x\\ \cline{2-4}&\\ mixture&y&0.30&0.3y \end{array}~\hfill \begin{cases} 75+x=y\\\\ 7.5+0.55x=0.3y \end{cases} \\\\[-0.35em] ~\dotfill](https://img.qammunity.org/2023/formulas/mathematics/high-school/4vbakjwa4mkron2ycu6gzcf1w7ebnir5h8.png)