Answer:
9.4 × 10²³ atoms
Step-by-step explanation:
To find the number of entities in a given substance we use the formula
N = n × L
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
However we weren't given the number of moles only the mass of oxygen was given. we can find the number of moles from that by using the formula
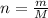
m is the mass
M is the molar mass
n is the number of moles
Molar mass of oxygen = 16 g/mol
mass in question = 25 g
We have
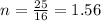
number of moles = 1.56 mol
The number of oxygen atoms is equal to
N = 1.56 × 6.02 × 10²³ = 9.3912 × 10²³
We have the final answer as
9.4 × 10²³ oxygen atoms
Hope this helps you