The question requires us to calculate the volume of hydrogen gas in a balloon, given the number of moles of hydrogen, temperature and pressure in the balloon.
We can collect the following information from the question:
number of moles of H2 = n = 0.24 mol
temperature = T = 35°C
pressure = P = 1.05 atm
Since we need to calculate the volume of a gas and the temperature and pressure given are not the under the STP (Standard Temperature and Pressure), we'll need to apply the Ideal Gas Law equation:
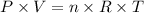
Rearranging the equation above to find volume (V), we have:
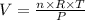
where n is the number of moles of hydrogen gas, T and P are the temperature and pressure given, and R is a the constant of gases (R = 0.0821 L.atm/mol.K)
Note that we need to use the units following the constant of gases - L for volume, atm for pressure, mol for number of moles and K for temperature. Thus, we need to convert the temperature given (35°C) into Kelvin degrees:
T = 35 + 273.15 = 308.15 K
Now that we have all required variables to calculate the volume, we replace them in the equation:
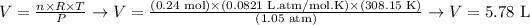
Therefore, the volume of hydrogen in the ballon, under the given temperature and pressure, is 5.78 L.
As the question requires the answer expressed with two signifcant figures, we can write that the volume of hydrogen is 5.8 L.