The first step we have to follow is to find the molar mass of FeCl2 using the atomic masses of each element:
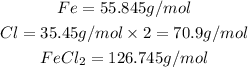
It means that each mole of FeCl2 has a mass of 126.745g/mol.
Use the molar mass to find the mass of the given amount of moles:
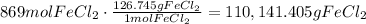
It means that there are 110,141.405 grams of FeCl2.
In scientific notation it would be 1.10x10^5g.