Using the following equation:
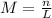
Where M represents the concentration (Molarity), n represents the number of moles of solute and L represents the liters of solution.
If we replace the values of the problem:
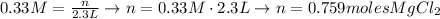
To find the number of grams, what we do is to multiply by the molar mass of MgCl2, which is 95.211g:
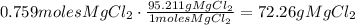
Therefore, 72.26gMgCl2 are needed.