The perimeter of a rectangle can be calculated using the following formula:
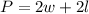
Where
"w" represents the width of the rectangle
"l" represents the length of the rectangle
If you know the perimeter and length of a rectangle, you can use the formula to determine the width. The first step is to write the formula for the width:
-Pass 2l to the left side of the expression by applying the opposite operation to both sides of it:
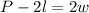
-Divide both sides by 2
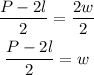
Now that we have determined a formula for w, replace the value of the length and perimeter and calculate the width:
P=84in
l=28in
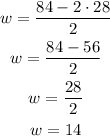
The width of the rectangular frame is 14 inches long