ANSWER
T
Step-by-step explanation
Given that;
The mass of the object is 9.6 grams
The volume of the object is 3.2mL
Follow the steps below to find the density of the object
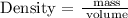
Substitute the given data into the formula above
Convert the volume of the object to cm^3
Recall, that 1mL is equivalent to 1cm^3
Hence, 3.2mL is 3.2 cm^3
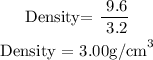
Therefore, the density of the object is 3.00 g/mL