In areaction, the number of moles consumed and produced have the same ratio as the coefficients on the balanced equation.
So, to calculate the number of moles of any chemical on the reaction given the number of moles of any other, we can use a rule of three.
We put the compound or atom we want on the first column and the one we have information of on the second, and their coefficients on the second row:
We want Al and we have information about Al₂O₃, and their coefficients on the balanced equation are 4 and 2, so:
Al --- Al₂O₃
4 2
Now, we use thise placements to write the equation:
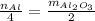
Where the n stands for the number of moles. Now we solve for Al:
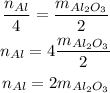
And since we know the number of moles of Al₂O₃, we have:
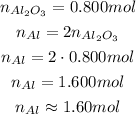
Where the last step we did an approximateion to get to 3 significant figures.
So, the number of moles of Al consumed is 1.60 mol.