Answer:
specific heat capacity = 5 J/kg °C
Step-by-step explanation:
To calculate the specific heat capacity of a substance, we can use the following formula:
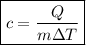 ,
,
where:
• c = specific heat capacity
• Q = heat energy
• m = mass of substance
• ΔT = change in temperature
We know from the question that Q = 5200J, m = 2kg, and ΔT = 47 - 42 = 5°C.
Using this information as well as the formula above, we can calculate the specific heat capacity of the substance:
c =
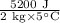
= 5 J/kg °C
Therefore the specific heat capacity of the substance is 5 J/kg °C.