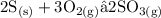Answer:
- Balancing equation must encounter the equality in moles of both reactants and products. (The left hand side must be equal to the right hand side)
- For the low concentration of oxygen, sulphurdioxide is produced and equation is balanced as below
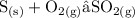
- For high oxygen concentration, sulphurtrioxide is produced and equation is balanced as below;