Answer:
Step-by-step explanation:
To find the ionic equation, first find all the ions that would be formed when the compounds dissolve in water.
the first (not ionic or balanced) equation is:
CrCl3 + Na2S > Cr2S3 + NaCl
Step 1, balancing
CrCl3 + Na2S > Cr2S3 + NaCl
turns into
2CrCl3 + 3Na2S > Cr2S3 + 6NaCl
Step 2, splitting into ions
CrCl3 splits into Cr{3+} and 3 Cl{-}, so 2CrCl3 splits into 2Cr{3+} and 6 Cl{-}
3Na2S splits into 6Na{+} and 3S{2-}
Cr2S3 does not split into anything since it is a solid
6NaCl into 6Na{+} and 6Cl{-}
All ions are aqueous
Step 3, making an ionic equation
Since the ion forms of the compounds are known, they can be replaced with or substituted into the ion forms
2CrCl3 + 3Na2S -> Cr2S3 + 6NaCl
turns into
2Cr{3+}(aq) + 6Cl{-}(aq) + 6 Na{+}(aq) + 3S{2-}(aq) -> Cr2S3(s) + 6Cl{-}(aq) + 6 Na{+}(aq)
This is the ionic equation
Step 4, canceling common ions out to find the net-ionic
Making a net-ionic equation is much like doing algebra, except the variables have been replaced with ions.
Since there is 6 Cl{-} on both sides, they cancel out, like how in the equation y + 3x = 7 + 3x, the 3x cancel out to make y = 7
Similarly, the 6 Na{+} cancel out.
The 2Cr{3+} and 3S{2-} don't cancel out since they are only present as ions on one side of the equation.
2Cr{3+} + 6Cl{-} + 6 Na{+} + 3S{2-} -> Cr2S3 + 6Cl{-} + 6 Na{+}
now turns into
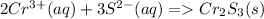
The first problem is now done.
I will go through the second question much quicker now, ask questions if you don't understand
1.
(NH4)2SO4 + AgClO4 -> Ag2SO4 + NH4ClO4
to
(NH4)2SO4 + 2AgClO4 -> Ag2SO4 + 2NH4ClO4
2.
(NH4)2SO4 to NH4+ and SO4{2-}
AgClO4 to Ag+ and ClO4-
Ag2SO4 is a solid so it does not split
NH4ClO4 to NH4+ and ClO4-
3.
2NH4+ + SO4{2-} + 2Ag+ + 2ClO4- ->Ag2SO4 + 2ClO4- + 2NH4+
this is the ionic equation
4. cancel out
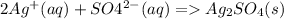
this is the net ionic equation