Answer:
Choice 3: (2, 1, -2, +1/2) is not allowed
Step-by-step explanation:
Let's first start with the definition the four quantum numbers
The four quantum numbers are (in order in which they appear in the parenthesis):
Number Symbol Possible
Values
Principal Quantum Number
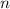
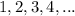 .
.
Angular Momentum QN
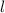
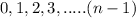
Magnetic QN
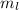
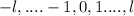
Spin Quantum Number
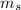
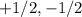
These are usually represented as
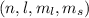 and in that order
and in that order
Note:
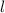 depends on the value of
depends on the value of
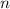
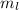 depends on the value of
depends on the value of
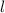
So to see which set is not allowed from the four choices given in the question, see which of these properties are violated.
Looking at the third choice we see that
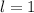 and
and
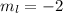
But
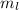 minimum value is -
minimum value is -
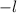 which should be -1 and nothing below that value Since -2 < -1,this property is violated and therefore is an invalid set
which should be -1 and nothing below that value Since -2 < -1,this property is violated and therefore is an invalid set
Answer: Choice 3: (2, 1, -2, +1/2) is not allowed